GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound 2c [PMID: 28613862] | Example 1166 [WO2015160641A2]
Compound class:
Synthetic organic
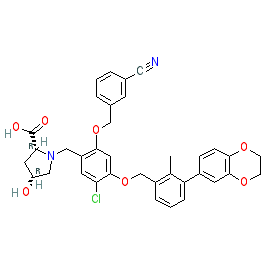
Comment: BMS-1166 is a small molecule that disrupts the PD-1/PD-L1 immune checkpoint interaction. It is the most potent of the [3-(2,3-Dihydro-1,4-benzodioxin-6-yl)-2-methylphenyl]methanol derivatives described in [2], and it is claimed as Example 1166 in Bristol-Myers Squibb's patent WO2015160641A2 [1]. NMR analysis suggests that BMS-1166 promotes the formation of dimeric PD-L1 in solution.
|
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
IUPAC Name  |
| (2R,4R)-1-[[5-chloro-2-[(3-cyanophenyl)methoxy]-4-[[3-(2,3-dihydro-1,4-benzodioxin-6-yl)-2-methylphenyl]methoxy]phenyl]methyl]-4-hydroxypyrrolidine-2-carboxylic acid |
Synonyms  |
| compound 2c [PMID: 28613862] | Example 1166 [WO2015160641A2] |
Database Links  |
|
| GtoPdb PubChem SID | 340590238 |
| PubChem CID | 118434635 |
| Search Google for chemical match using the InChIKey | QBXVXKRWOVBUDB-GRKNLSHJSA-N |
| Search Google for chemicals with the same backbone | QBXVXKRWOVBUDB |
| UniChem Compound Search for chemical match using the InChIKey | QBXVXKRWOVBUDB-GRKNLSHJSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | QBXVXKRWOVBUDB-GRKNLSHJSA-N |
Product suppliers
View disclaimer
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖|
BMS-1166 (links to external site)
Cat. No. HY-102011 |