GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
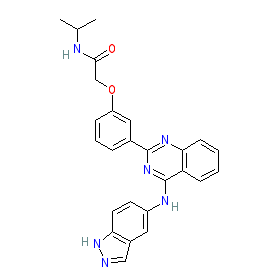
Synonyms: KD-025 | KD025 | Rezurock® | SLx 2119 | SLx2119
belumosudil is an approved drug (FDA (2021))
Compound class:
Synthetic organic
Comment: Belumosudil (KD025) is an oral and selective inhbitor of Rho associated coiled-coil containing protein kinase 2 (ROCK2) [1], with clinical anti-inflammatory/immunomodulatory activity.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| KD025 is a clinical candidate for inflammatory conditions including plaque psoriasis, graft vs. host disease and idiopathic pulmonary fibrosis. In vitro, it reduces IL-17 secretion in human peripheral blood mononuclear cells via an IL-1/IL-6-independent mechanism [5]. This evidence supported selective ROCK2 inhibition as a valid strategy for the treatment of IL-17-driven inflammatory diseases. |
| Immunopharmacology Disease | |||
| Disease | X-Refs | Comment | References |
| Idiopathic pulmonary fibrosis |
Disease Ontology:
DOID:0050156 Orphanet: ORPHA2032 |
Phase 2 clinical candidate for IPF (NCT02688647). | |
| Psoriasis |
Disease Ontology:
DOID:8893 |
Phase 2 clinical candidate for moderate to severe plaque psoriasis (NCT02852967). | |
| Graft versus host disease | FDA approved for this disorder in July 2021. | 2-3 | |