GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
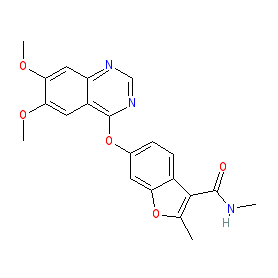
Synonyms: Elunate® | Fruzaqla® | HMPL-013 | HMPL013
fruquintinib is an approved drug (China (2018), FDA (2023), EMA (2024))
Compound class:
Synthetic organic
Comment: Fruquintinib (HMPL-013) is a potent and selective inhibitor of VEGFR 1-3 tyrosine kinases, that was developed for antineoplastic activity [1] (preclinical data is reported in this reference).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| HMPL-013 (as fruquintinib) is in Phase 3 clinical trial as monotherapy in patients with advanced non-small cell lung cancer (NSCLC; see NCT02691299) and Phase 2 for the same indication but in combination with the approved EGFR inhibitor gefitinib (see NCT02976116). In 2018 China's drug regulatory agency (NMPA) approved the use of fruquintinib to treat metastatic colorectal cancer that has been prevoiusly treated with at least 2 prior chemotherapy regimens, including those treated with anti-VEGF or anti-EGFR (WT) drugs. In November 2023 the FDA approved fruquintinib to treat highly pre-treated metastatic colorectal cancer. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02691299 | A Phase III Clinical Trial of Fruquintinib in Patients With Advanced Non-small Cell Lung Cancer | Phase 3 Interventional | Hutchison Medipharma Limited | ||
| NCT02976116 | A Phase II Study of Fruquintinib in Combination With Gefitinib in Patients With Advanced Non-small Cell Lung Cancer | Phase 2 Interventional | Hutchison Medipharma Limited | ||
| NCT02314819 | A Phase III Trial Evaluating Fruquintinib Efficacy and Safety in 3+ Line Colorectal Cancer Patients (FRESCO) | Phase 3 Interventional | Hutchison Medipharma Limited | ||
| NCT04322539 | A Study of Efficacy and Safety of Fruquintinib (HMPL-013) in Patients With Metastatic Colorectal Cancer | Phase 3 Interventional | Hutchison Medipharma Limited | Results from this study will inform the progress of fruquintinib's development outside of China. | |