GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: leuco-methylthioninium | LMTM | LMTX | TRx-0237 | TRx0237
leucomethylthioninium is an approved drug
Compound class:
Synthetic organic
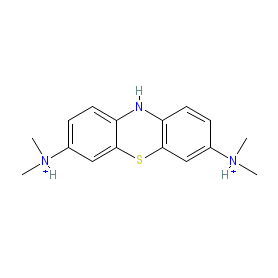
Comment: Leucomethylthioninium complexed with bromide or methane sulfonate (LMTX) was designed as a methylthioninium chloride (MTC, or methylene blue) analogue with improved pharmacokinetic properties [1]. MTC and LMTX are tau aggregation inhibitors with potential in Alzheimer's disease (AD) and other tau-opathies. We believe this is the compound being investigated in Phase 3 clinical trials as TRx0237.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Click here to link to a list of Phase 3 TRx0237 trials in patients with AD registered with ClinicalTrials.gov. Results presented at the Alzheimer's Association International Conference in Toronto (July 2016) and reported on the NewScientist website, suggest encouraging results in the sub-cohort of patients with mild-moderate AD, but not taking other anti-AD drugs. This group of patients exhibited improved cognitive and functional outcomes as well as a slowing of brain atrophy. These are preliminary findings, as yet unpublished in a peer-reviewed article. The Chinese drug regulator (NMPA) approved leucomethylthioninium (as methylthioninium chloride; Lumeblue®) in 2024, as an imaging reagent to enhance visualisation of colorectal lesions. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Most neurodegenerative diseases are characterised by deposition of aberrantly processed and aggregated proteins, e.g. extraneuronal deposition of amyloid β and microtubule-associated protein tau in Alzheimer's plaques and filaments respectively. Inhibition of the formation of tau filaments/tangles is one strategy being evaluated for potential clinical effect in tauopathies [2]. Leucomethylthioninium is one such tau aggregation inhibitor. |