GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
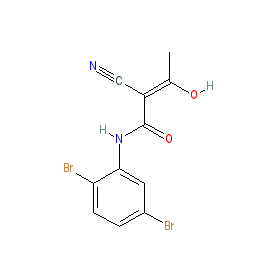
Synonyms: α-cyano-beta-hydroxy-β-methyl-N-(2, 5-dibromophenyl)propenamide | alpha-cyano-beta-hydroxy-beta-methyl-N-(2, 5-dibromophenyl)propenamide
Compound class:
Synthetic organic
Comment: LFM-A13 is a potent, cell-permeable, reversible, substrate competitive, and specific inhibitor of Bruton's tyrosine kinase (BTK) [1]. It was the first reported BTK-selective tyrosine kinase inhibitor and the first anti-leukemic agent targeting BTK. Investigated preclinically for B-cell non-Hodgkin's lymphoma [5-6].
Structurally LFM-A13 is an analogue of a metabolite of the antirheumatic drug leflunomide. The Mahajan et al. article also reports a three-dimensional homology model suggesting an energetically favorable position of LFM-A13 docked within BTK's catalytic site [1]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Mahajan et al. (1999) report that LFM-A13 did not inhibit JAK1, JAK3, IRK, EGFR, or HCK in their assays [1]. This article also publishes a Ki of ~2500nM for LFM-A13 vs. BTK expressed in a baculovirus system and immunoprecipitated from NALM-6 human B-lineage ALL cells. LFM-A13 sensitizes DT40 lymphoma B cells to vincristine- or ceramide-induced apoptosis [1]. Later studies report polo-like kinase inhibition by LFM-A13 (IC50 6100nM vs. human PLK3) with in vitro and in vivo anti-proliferative activity against human breast cancer [3-4], in addition to its chemosensitizing activity against human leukemic B-cell precursors [2]. |
| Selectivity at enzymes | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||