GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
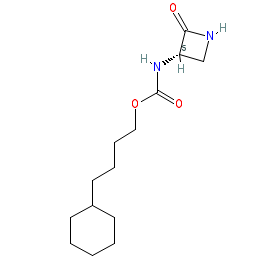
Synonyms: ARN-726 | compound 6 [PMID: 25874594]
Compound class:
Synthetic organic
Comment: ARN726 is from a series of β-lactam derivatives that are potent, selective, and systemically active inhibitors of intracellular N-Acylethanolamine acid amidase (NAAA) by covalently binding the enzyme's catalytic cysteine. It exhibits anti-inflammatory effects in both mouse models and human macrophages [2]. In the corresponding patent [1] the structure seems to be example 5. There is a patchy SAR table for 106 structures on page 104 of the WO2014144836 PDF. The R isomer of this structure PubChem CID 86282216 is reported to be less active.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Piomelli D. et al.. (2014)
Carbamate derivatives of lactam based n-acylethanolamine acid amidase (NAAA) inhibitors. Patent number: WO2014144836. Assignee: Regents Of The University Of California. Priority date: 15/03/2013. Publication date: 18/09/2014. |
|
2. Ribeiro A, Pontis S, Mengatto L, Armirotti A, Chiurchiù V, Capurro V, Fiasella A, Nuzzi A, Romeo E, Moreno-Sanz G et al.. (2015)
A Potent Systemically Active N-Acylethanolamine Acid Amidase Inhibitor that Suppresses Inflammation and Human Macrophage Activation. ACS Chem Biol, 10 (8): 1838-46. [PMID:25874594] |