GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: DCC-2618 | DCC2618 | Quinlock®
ripretinib is an approved drug (FDA (2020), EMA (2021))
Compound class:
Synthetic organic
Comment: Ripretinib (DCC-2618) is an orally bioavailable, potent pan-KIT and PDGFRα kinase inhibitor that was developed by Dicephera Pharmaceuticals. It was designed for the treatment of malignancies that are driven by KIT and/or PDGFRα, for example gastrointestinal stromal tumors, glioblastoma multiforme and systemic mastocytosis. Ripretinib has activity across a broad range of resistance mutations in its target kinases, that emerge in response to imatinib treatment. Ripretinib binds to the 'switch pocket' of KIT, a domain that regulates the enzyme's catalytic conformation. Compounds that act in this way are often referred to as switch control inhibitors [1]. Another example of a switch control inhibitor is avapritinib.
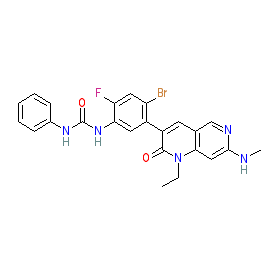
The chemical structure shown here matches that supplied to the WHO for the INN, and this is claimed in patent WO2010051373 [2] as Example 39. Note that some PubChem SIDs are linked to an incorrect structure (as represented in PubChem CID 46208890). |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| The US FDA granted ripretinib (DCC-2618) orphan drug designation (ODD) for gastrointestinal stromal tumours (GIST) in 2014. EMA ODD for the same indication followed in 2017. Following successful progress through clinical trials in patients with advanced GIST, the FDA authorised use for this indication in May 2020 (based on evidence from clinical trial NCT03353753). This approval permits ripretinib's use in patients who have received ≥3 kinase inhibitor regimens, including imatinib therapy. Click here to link to ClinicalTrials.gov's full list of DCC-2618 studies. |