GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
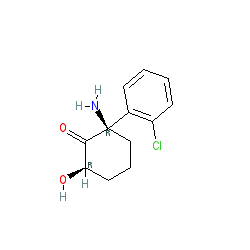
Synonyms: (2R,6R)HNK
Compound class:
Synthetic organic
Comment: A recent (April 2016) report [1] shows that the metabolism of racemic ketamine to hydroxynorketamine (HNK) is essential for its antidepressant effects, and that these reside in this entry as the (2R,6R)-HNK enantiomer. In mice these actions are independent of NMDAR inhibition and lack ketamine-related side effects. The (flat) rendering of HNK is PubChem CID 133669. The (2S,6S)-HNK is represented as PubChem CID 71519584 but this showed a decreased antidepressant effect compared to (2R,6R)-HNK.
|
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
IUPAC Name  |
| (2R,6R)-2-amino-2-(2-chlorophenyl)-6-hydroxycyclohexan-1-one |
Synonyms  |
| (2R,6R)HNK |
Database Links  |
|
| GtoPdb PubChem SID | 315661239 |
| PubChem CID | 89504167 |
| Search Google for chemical match using the InChIKey | CFBVGSWSOJBYGC-ZYHUDNBSSA-N |
| Search Google for chemicals with the same backbone | CFBVGSWSOJBYGC |
| UniChem Compound Search for chemical match using the InChIKey | CFBVGSWSOJBYGC-ZYHUDNBSSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | CFBVGSWSOJBYGC-ZYHUDNBSSA-N |