GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
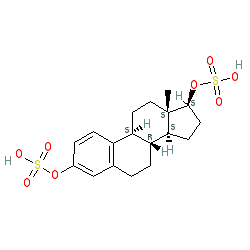
Synonyms: 17beta-estradiol-3,17-disulfate | estradiol-3,17-disulfate
Compound class:
Synthetic organic
Comment: 17Β-estradiol is the most potent mammalian estrogenic steroid. This is the sulphated form of that compound.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Estradiol disulfate is reported to inhibit transport of dehydroepiandrosterone (DHEA) by multidrug resistance-associated protein 4 (ABCC4) [1]. |
| Selectivity at transporters | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||