GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
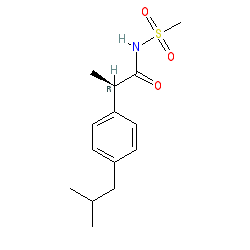
Synonyms: DF 1681Y | repertaxin
Compound class:
Synthetic organic
Comment: Reparixin is an orally bioavailable compound that has been explored for potential to disrupt IL-8/CXCR1/2 signalling in cancer and transplant indications. It negatively modulates CXCR1 and CXCR2 dependent functions [1], but without inhibiting chemokine binding to either receptor. Direct binding to CXCR1 has only been indicated by molecular modelling, so the mechanism of action of this molecule remains to be fully resolved.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Reparixin has completed Phase 3 clinical trial as a single agent anti-rejection therapy for patients receiving pancreatic islet transplant . Published results showed that it did not improve insulin secretion in islet cell recipients [3]. Phase 2 trial NCT01861054 in HER2 negative breast cancer has reported that as a single agent reparixin reduced cancer stem cell numbers, with no serious adverse effects [2]. A Phase 2 study investigating reparixin's potential to prevent early allograft dysfunction in orthotopic liver transplantation (NCT03031470) has been completed, but no results have been published as far as we can ascertain. COVID-19: Reparixin's anti-inflammatory activity is being explored for potential to reduce the risk of disease progression in COVID-19 patients. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05254990 | Reparixin as add-on Therapy to Standard of Care to Limit Disease Progression in Adult Patients With COVID-19. | Phase 3 Interventional | Dompé Farmaceutici S.p.A | ||