GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
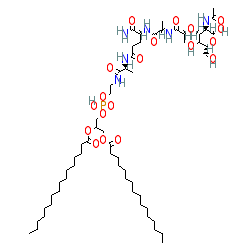
Synonyms: CGP 19835 | CGP-19835 | L-MTP-PE | liposomal muramyl tripeptide phosphatidyl ethanolamine | Mepact® | MLV 19835A
mifamurtide is an approved drug (EMA (2009))
Compound class:
Synthetic organic
Comment: Mifamurtide is a synthetic analogue of the bacterial cell wall component, muramyl dipeptide (MDP). MDP stimulates the immune system via activation of microbial pattern recognition receptors such as TLR4 and NOD2 [3,9].

View more information in the IUPHAR Pharmacology Education Project: mifamurtide |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| The EMA granted mifamurtide orphan drug designation for the treatment of osteosarcoma in children and young adults in 2004. The drug was subsequently fully approved by the EMA in 2009. Mifamurtide is indicated for the treatment of high-grade, nonmetastasizing, resectable osteosarcoma following complete surgical removal and/or in combination with chemotherapy. Further reading: [1-2,4,6]. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Mifamurtide activates the pattern recognition receptor NOD2 (gene symbol NLRC2) on monocytes and macrophages, stimulating the production of pro-inflammatory cytokines. This activates an antibacterial-like immune response which kills cancer cells [5,7]. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) European Medicines Agency (EMA) |