GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
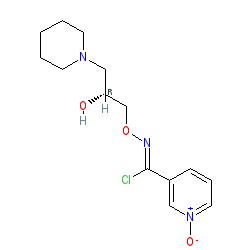
Synonyms: BRX-220 | BRX220 | Miplyffa®
arimoclomol is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: Arimoclomol is an oral therapeutic candidate for the treatment of amyotrophic lateral sclerosis (ALS). Arimoclomol may be delivered as the citrate salt (BRX-345 with PubChem CID 72710735)
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| The EMA and FDA have granted arimoclomol orphan drug status for the treatment of the neurodegenerative disease amyotrophic lateral sclerosis (ALS, aka Lou Gehrig's disease). A Phase 2/III clinical trial assessing arimoclomol in ALS (NCT00706147) is ongoing. The FDA approved arimoclomol in September 2024, to treat the neurological manifestations of the ultra-rare progressive and fatal neurodegenerative condition Niemann-Pick disease type C in combination with miglustat (a glucosylceramide synthase inhibitor) [8]. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Arimoclomol acts as a coinducer of heat shock proteins (Hsps) [3] and this action has been reported to slow the process of motor neuron death and extend life in cellular and whole animal models of ALS [2,4-6,9]. Arimoclomol is reported to prolong the activation of heat shock transcription factor 1 (HSF1; Q00613), the master regulator of stress-inducible Hsps, including Hsp70 and Hsp90 [3]. Increased Hsp70 expression is believed to be responsible for the neuroprotective effect of arimoclomol in ALS mice [7]. In Niemann-Pick disease type C (NPC) lysosomes are damaged by accumulation of unesterified cholesterol due to misfolded NPC protein (NPC1). Arimoclomol appears to improve lysosome function via different pathways; increased activation of transcription factors (EB/TFEB and E3/TFE3) and upregulated expression of coordinated lysosomal expression and regulation (CLEAR [10]) genes; amplified heat shock protein (stress) response to misfolded NPC protein to improve transport of unesterified cholesterol out of lysosomes. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT00706147 | Phase II/III Randomized, Placebo-controlled Trial of Arimoclomol in SOD1 Positive Familial Amyotrophic Lateral Sclerosis | Phase 2/Phase 3 Interventional | University of Miami | ||
| NCT03491462 | Arimoclomol in Amyotropic Lateral Sclerosis | Phase 3 Interventional | ZevraDenmark | 1 | |
| NCT02612129 | Arimoclomol Prospective Study in Participants Diagnosed With Niemann-Pick Disease Type C | Phase 2/Phase 3 Interventional | ZevraDenmark | ||