GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
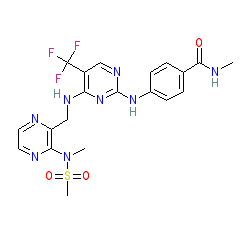
Synonyms: Avmapki Fakzynja (avutometinib + defactinib co-pack) | PF-04554878 | VS-6063
defactinib is an approved drug
Compound class:
Synthetic organic
Comment: Defactinib (VS-6063) is an orally bioavailable, second generation, small-molecule focal adhesion kinase (FAK) inhibitor with potential antiangiogenic and antineoplastic activities [1,9].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Golubovskaya VM, Cance WG. (2007)
Focal adhesion kinase and p53 signaling in cancer cells. Int Rev Cytol, 263: 103-53. [PMID:17725966] |
|
2. Grisham R, Monk BJ, Van Nieuwenhuysen E, Moore KN, Fabbro M, O'Malley DM, Oaknin A, Thaker P, Oza AM, Colombo N et al.. (2025)
GOG-3097/ENGOT-ov81/GTG-UK/RAMP 301: a phase 3, randomized trial evaluating avutometinib plus defactinib compared with investigator's choice of treatment in patients with recurrent low grade serous ovarian cancer. Int J Gynecol Cancer, 35 (11): 101832. [PMID:39375168] |
|
3. Jones SF, Siu LL, Bendell JC, Cleary JM, Razak AR, Infante JR, Pandya SS, Bedard PL, Pierce KJ, Houk B et al.. (2015)
A phase I study of VS-6063, a second-generation focal adhesion kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs, 33 (5): 1100-7. [PMID:26334219] |
|
4. Kang Y, Hu W, Ivan C, Dalton HJ, Miyake T, Pecot CV, Zand B, Liu T, Huang J, Jennings NB et al.. (2013)
Role of focal adhesion kinase in regulating YB-1-mediated paclitaxel resistance in ovarian cancer. J Natl Cancer Inst, 105 (19): 1485-95. [PMID:24062525] |
|
5. Klaeger S, Heinzlmeir S, Wilhelm M, Polzer H, Vick B, Koenig PA, Reinecke M, Ruprecht B, Petzoldt S, Meng C et al.. (2017)
The target landscape of clinical kinase drugs. Science, 358 (6367). [PMID:29191878] |
|
6. Lin HM, Lee BY, Castillo L, Spielman C, Grogan J, Yeung NK, Kench JG, Stricker PD, Haynes AM, Centenera MM et al.. (2018)
Effect of FAK inhibitor VS-6063 (defactinib) on docetaxel efficacy in prostate cancer. Prostate, 78 (4): 308-317. [PMID:29314097] |
|
7. Owens LV, Xu L, Dent GA, Yang X, Sturge GC, Craven RJ, Cance WG. (1996)
Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann Surg Oncol, 3 (1): 100-5. [PMID:8770310] |
|
8. Polte TR, Hanks SK. (1995)
Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci USA, 92 (23): 10678-82. [PMID:7479864] |
|
9. Provenzano PP, Keely PJ. (2009)
The role of focal adhesion kinase in tumor initiation and progression. Cell Adh Migr, 3 (4): 347-50. [PMID:19690467] |
|
10. Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. (1994)
Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol, 14 (3): 1680-8. [PMID:7509446] |
|
11. Shimizu T, Fukuoka K, Takeda M, Iwasa T, Yoshida T, Horobin J, Keegan M, Vaickus L, Chavan A, Padval M et al.. (2016)
A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 77 (5): 997-1003. [PMID:27025608] |
|
12. Zauderer MG, Jegede O, Jackman DM, Zwiebel JA, Gray RJ, Wang V, McShane LM, Rubinstein LV, Patton DR, Williams PM et al.. (2024)
Phase II Study of Defactinib (VS6063) in Patients With Tumors With NF2 Loss: Results From the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol U. JCO Precis Oncol, 8: e2400327. [PMID:39693587] |