GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Avmapki Fakzynja (avutometinib + defactinib co-pack) | PF-04554878 | VS-6063
defactinib is an approved drug
Compound class:
Synthetic organic
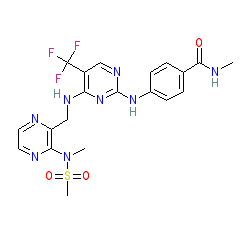
Comment: Defactinib (VS-6063) is an orally bioavailable, second generation, small-molecule focal adhesion kinase (FAK) inhibitor with potential antiangiogenic and antineoplastic activities [1,9].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Defactinib (PF-04554878/VS-6063) was progressed to clinical evaluations in a variety of advanced solid malignancies [3,11]. The EMA issued orphan drug designations for the treatment of malignant mesothelioma and ovarian cancers in 2013 and 2015 respectively. The FDA granted accelerated approval for the combination of avutometinib and defactinib in May 2025, indicated to treat KRAS-mutated recurrent low-grade serous ovarian cancer (following previous systemic therapy) |
Mechanism Of Action and Pharmacodynamic Effects  |
| The tyrosine kinase FAK is a key signal transducer for integrins and growth factors, that is upregulated in many tumor cell types and is involved in tumor cell invasion, migration and proliferation [7]. Autophosphorylation of FAK at Tyr397 mediates interation with downstream signalling molecules including ERK, JNK/MAPK and PI3K/Akt [8,10]. FAK inhibition is therefore likely to prevent the activation of these signal transduction pathways, thus inhibiting tumor cell migration, proliferation and survival [1]. In addition, FAK inhibition with defactinib has been reported to overcome YB-1 (YBX1; P67809)-mediated paclitaxel resistance in in vitro tumour cell models, via an Akt-dependent pathway [4]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT06369259 | Open-label Phase 2 Study of Avutometinib (RAF/MEK Clamp) in Combination With Defactinib (FAK Inhibitor) and Cetuximab in Patients With Unresectable, Anti-EGFR-Refractory Advanced Colorectal Cancer | Phase 2 Interventional | M.D. Anderson Cancer Center | ||
| NCT06394804 | A Study of Avutometinib, Defactinib, and Letrozole in People With Low-Grade Serous Ovarian Cancer | Phase 2 Interventional | Memorial Sloan Kettering Cancer Center | ||
| NCT02465060 | Targeted Therapy Directed by Genetic Testing in Treating Patients With Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma (The MATCH Screening Trial) | Phase 2 Interventional | National Cancer Institute (NCI) | 12 | |
| NCT06072781 | A Study of Avutometinib (VS-6766) + Defactinib (VS-6063) in Recurrent Low-Grade Serous Ovarian Cancer | Phase 3 Interventional | Verastem, Inc. | 2 | |