GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
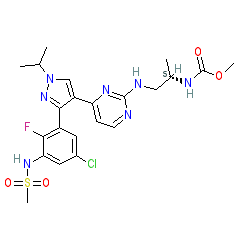
Synonyms: Braftovi® | LGX-818 | LGX818
encorafenib is an approved drug (FDA and EMA (2018))
Compound class:
Synthetic organic
Comment: Encorafenib (LGX-818) is an orally available BRAF kinase inhibitor with antineoplastic activity.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Encorafenib received FDA approval as a treatment for advanced BRAF-mutant melanoma in June 2018. The approval is for a combination encorafenib plus the MEK1/2 kinase inhibitor, binimetinib. Results from Phase 3 clinical trial NCT01909453 which compared the combination of encorafenib plus binimetinib and encorafenib monotherapy to vemurafenib monotherapy in BRAF mutant melanoma were published in May 2018 [1]. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |