GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: AHU377 | Entresto® | Neparvis®
sacubitril is an approved drug (EMA & FDA (2015))
Compound class:
Synthetic organic
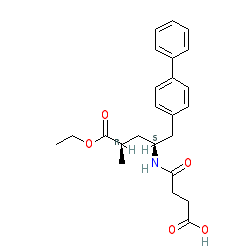
Comment: Sacubitril is a prodrug activated by de-ethylation via esterases to the neprilysin inhibitor sacubitrilat. Neprilysin is also known as neutral endopeptidase (NEP), and its official gene symbol is MME (membrane metallo-endopeptidase ). The chemical database relationships are detailed in this blogpost. Sabucatril is a component of the approved fixed mixture Entresto®, previously known as LCZ696, and which is now approved for heart failure (see Clinical data tab).
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| In 2015, the EMA and FDA approved the fixed-dose combination Entresto® (sacubitril plus valsartan) to treat heart failure. Sacubitril is not approved as a monotherapy. Entresto® is reported to be superior to enalapril in reducing the risks of death and hospitalization for heart failure [1]. |