GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: R 788 | R-788 | R7935788 | Tavalisse® | Tavlesse®
fostamatinib is an approved drug (FDA (2018), EMA (2020))
Compound class:
Synthetic organic
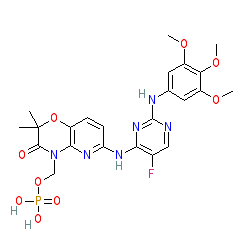
Comment: Fostamatinib is a spleen tyrosine kinase (Syk) inhibitor prodrug. Fostamatinib is administered orally in disodium salt preparations (e.g. PubChem CID 25008120 and fostamatinib disodium hexahydrate CID 24828759). It is rapidly converted to tamatinib by alkaline phosphatases in intestinal enterocytes [6]. Although development in rheumatoid arthritis was discontinued in 2013 due to a lack of observed efficacy, development continued in patients with chronic and persistent immune thrombocytopenia (ITP).
|
|
|||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Fostamatinib is the first selective Syk inhibitor to receive clinical approval, and this approval is for treatment of an autoimmune disorder. |
| Immunopharmacology Disease | |||
| Disease | X-Refs | Comment | References |
| Rheumatoid arthritis |
Disease Ontology:
DOID:7148 OMIM: 180300 |
Phase 3 clinical candidate for RA. | 2 |
| Autoimmune thrombocytopenic purpura |
Disease Ontology:
DOID:8924 OMIM: 188030 Orphanet: ORPHA3002 |
Approved drug for ITP. FDA approval granted in April 2018. | |