GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
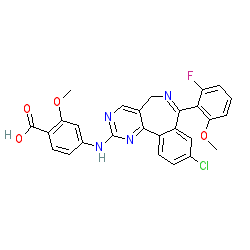
Synonyms: MLN-8237
Compound class:
Synthetic organic
Comment: Alisertib is a second generation, orally bioavailable and selective Aurora kinase A inhibitor.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Alisertib was in Phase 2I clinical development for the treatment of relapsed/refractory peripheral T-cell lymphoma (NCT01482962), but this study has been terminated (May 2015). A Phase 2 study for unresectable stage III-IV melanoma (NCT01316692) is still ongoing. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Since Aurora kinase A localises to spindle poles and spindle microtubules during mitosis, it is thought to regulate spindle assembly. Aberrant expression of Aurora kinases occurs in a wide variety of cancers, including colon and breast cancers. Inhibition of Aurora kinase A by alicertib appears to disrupt the assembly of the mitotic spindle apparatus culminating in the reduced cellular proliferation [1,3]. |