GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
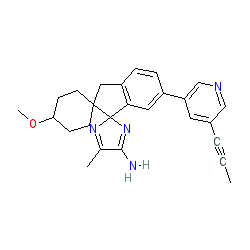
Synonyms: AZD 3293 | AZD-3293 | LY3314814
Compound class:
Synthetic organic
Comment: Lanabecestat (AZD3293) is an oral, potent and selective small molecule inhibitor of BACE1 being developed by AstraZeneca and Eli Lilly [4] . It is now proceeding to Phase III trials [3]. Note our initial chemical assignment was based on the crystallisation data in US20140031379 [2] but the name is now attached to two CIDs, the one designated in this entry with correct stereoisomer and the flat form as PubChem CID 57404290
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Results from Phase 1 study NCT01795339 show significantly and dose-dependently reduced levels of amyloid beta in the cerebro-spinal fluid of Alzheimer’s patients and healthy volunteers [5]. Phase II/III study NCT02245737 is underway in patients with early Alzheimer´s disease. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01795339 | A Two-part Multiple Dose Study to Assess the Safety and Effects of AZD3293 in Healthy Elderly and Alzheimer's Patients | Phase 1 Interventional | AstraZeneca | ||
| NCT02245737 | An Efficacy and Safety Study of Lanabecestat (LY3314814) in Early Alzheimer's Disease | Phase 2/Phase 3 Interventional | AstraZeneca | ||