GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
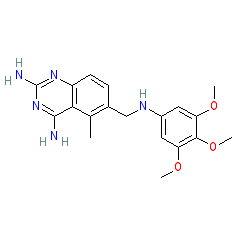
Synonyms: CI-898 | Neutrexin® | trimetrexate glucuronate
trimetrexate is an approved drug (FDA (1993))
Compound class:
Synthetic organic
Comment: Trimetrexate is a quinazoline derivative dihydrofolate reductase inhibitor [6]. Chemically it is a methotrexate (MTX) analogue.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Trimetrexate was originally approved by the US FDA in 1993, but use of this drug has since been discontinued. Trimetrexate was used in combination with folinic acid to treat moderate to severe Pneumocystis carinii pneumonia (PCP) in patients with compromised immune systems [3]. It was also used to treat some types of cancer including colon cancer [5] and investigated as a treatment for leiomyosarcoma [4] and methotrexate resistant skin lymphoma [2]. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Trimetrexate inhibits dihydrofolate reductase (DHFR) [6] across many species. Inhibition of this enzyme reduces active coenzyme tetrahydrofolate and folic acid supplies within cells (be they host cells or infection causing microbes). This leads to reduced thymidylate biosynthesis, and inhibition of folate-dependent formyltransferases. The net effect is disruption of DNA, RNA, and protein synthesis and this leads to cell death. |