GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: RTA 408 | RTA-408 | Skyclarys®
omaveloxolone is an approved drug (FDA (2023), EMA (2024))
Compound class:
Synthetic organic
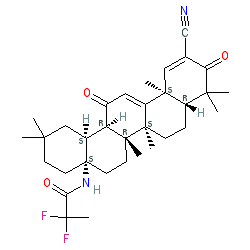
Comment: Chemically omaveloxolone (RTA 408) is a second generation synthetic oleanane triterpenoid compound, which structurally resembles bardoxolone, and has difluoropropanamide replacing the carboxylic acid moiety.
This compound is claimed in patent WO2013163344A1. The chemical structure represented above, was drawn from this patent, but structures with alternate stereochemistry are submitted in PubChem. Pharmacologically omaveloxolone activates the Nrf2 transcription factor, to modulate expression of proteins that have antioxidative, anti-inflammatory, and mitochondrial bioenergetic activities [6-7]. |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Omaveloxolone (RTA 408) was investigated for potential to treat a number of conditions, including post-surgical ocular pain/inflammation, dermatological disease eg radiation-induced dermatitis [8] and non-small-cell lung cancer [9]. For a full list of trials registered at ClinicalTrials.gov, click here. Omaveloxolone was issued with Orphan Drug [2], Fast Track, and Rare Pediatric Disease Designations from the FDA, as a treatment for the ultra-rare, neurodegenerative, and life-limiting disorder Friedreich's ataxia. The EMA also designated omaveloxolone as an orphan drug for this disease [1]. The FDA approved omaveloxolone, as the first therapy for this indication, in February 2023 [6]. |
Mechanism Of Action and Pharmacodynamic Effects  |
| RTA 408 activates the cytoprotective transcription factor Nrf2 (NFE2L2, Q16236) [3] in rat skin and induces expression of cytoprotective genes [9]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02259231 | RTA 408 Capsules in Patients With Melanoma - REVEAL | Phase 1/Phase 2 Interventional | Reata Pharmaceuticals, Inc. | ||
| NCT02255435 | RTA 408 Capsules in Patients With Friedreich's Ataxia - MOXIe | Phase 2 Interventional | Reata Pharmaceuticals, Inc. | 4-5 | |