GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: HOE-901 | HOE901 | Lantus®
insulin glargine is an approved drug (FDA and EMA (2000))
Compound class:
Peptide
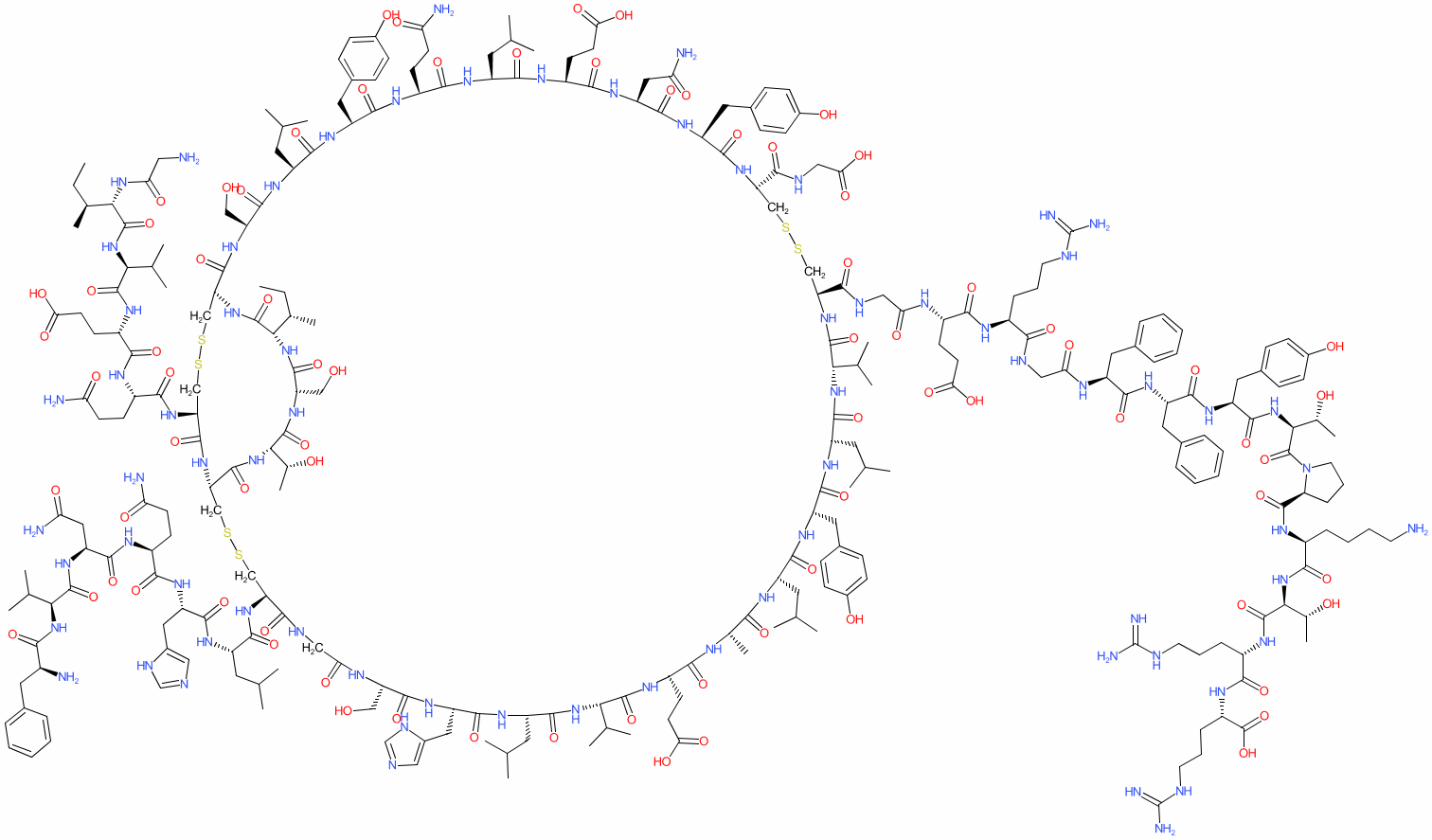
Comment: Insulin glargine is a recombinant, modified analogue of human insulin. The chemical structure shown here was generated using the SMILES from PubChem CID 44146714.
A number of insulin glargine biosimilars have been approved internationally since the expiration of patents on Lantus® in 2014: a comprehensive list that is maintained by the Generics and Biosimilar Initiative (https://www.gabionline.net) is available here. The first fully interchangeable biosimilar to be FDA approved was Semglee® (insulin glargine-yfgn; Mylan Pharmaceuticals), in July 2021. 
View more information in the IUPHAR Pharmacology Education Project: glargine |
|
|||||||||||||||
| Chemical Modification | |
| The A chain has peptide sequence GIVEQCCTSICSLYQLENYCG, where the C terminal glycine replaces the natural asparagine. The B chain has the sequence FVNQHLCGSHLVEALYLVCGERGFFYTPKTRR which has two additional C-terminal arginine residues compared to endogenous insulin. Disulphide bond formation is equivalent to that observed in the endogenous active hormone dimer. | |
Download 2D Structure  |
|
| Canonical SMILES | Download |
| Isomeric SMILES | Download |
Molecular structure representations generated using Open Babel