GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
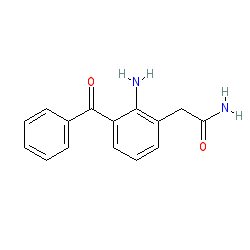
Synonyms: AHR-9434 | AL-6515 | Nevanac®
nepafenac is an approved drug (FDA (2005), EMA (2007))
Compound class:
Synthetic organic
Comment: Nepafenac is a non-steroidal anti-inflammatory drug (NSAID).

View more information in the IUPHAR Pharmacology Education Project: nepafenac |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Gamache DA, Graff G, Brady MT, Spellman JM, Yanni JM. (2000)
Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation, 24 (4): 357-70. [PMID:10850857] |
|
2. Jones BM, Neville MW. (2013)
Nepafenac: an ophthalmic nonsteroidal antiinflammatory drug for pain after cataract surgery. Ann Pharmacother, 47 (6): 892-6. [PMID:23715071] |
|
3. Ke TL, Graff G, Spellman JM, Yanni JM. (2000)
Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation, 24 (4): 371-84. [PMID:10850858] |
|
4. Lane SS. (2006)
Nepafenac: a unique nonsteroidal prodrug. Int Ophthalmol Clin, 46 (4): 13-20. [PMID:17060788] |
|
5. Takahashi K, Saishin Y, Saishin Y, Mori K, Ando A, Yamamoto S, Oshima Y, Nambu H, Melia MB, Bingaman DP et al.. (2003)
Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci, 44 (1): 409-15. [PMID:12506103] |
|
6. Zanetti FR, Fulco EA, Chaves FR, da Costa Pinto AP, Arieta CE, Lira RP. (2012)
Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery: a randomized trial. Indian J Ophthalmol, 60 (4): 277-81. [PMID:22824596] |