GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
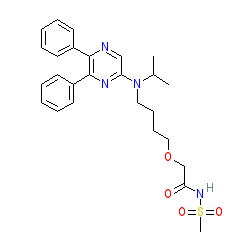
Synonyms: ACT-293987 | NS-304 | Uptravi®
selexipag is an approved drug (FDA (2015), EMA (2016))
Compound class:
Synthetic organic
Comment: Selexipag represents a first-in-class selective, long-acting, oral prostacyclin IP receptor agonist. This compound is a prodrug, with the active molecule being MRE-269.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Following successful Phase 3 trial this compund has been approved by the US FDA for the treatment of pulmonary arterial hypertension [5]. Click here to link to ClinicalTrials.gov's complete list of selexipag trials. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Prostacyclin is an endogenous vascular endothelial factor with an important role in vascular homeostasis as an inhibitor of platelet aggregation and a potent vasodilator [3]. The physiological functions are mediated by the activation of the PGI2 receptor (IP receptor) [4]. IP receptor agonists are being pursued as clinical therapeutics for control of platelet and vascular function. Selexipag is one such agonist of the PGI2 prostaglandin receptor. This action causes vasodilation in the pulmonary circulation, therby reducing pulmonary arterial blood pressure. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |