GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
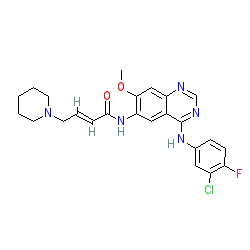
Synonyms: PF-00299804 | PF00299804 | Vizimpro®
dacomitinib is an approved drug (FDA (2018), EMA (2019))
Compound class:
Synthetic organic
Comment: Dacomitinib is a second-generation, irreversible pan inhibitor of HER tyrosine kinases (EGFR, and ERBBs) that was developed by Pfizer.
Results from the ARCHER 1050 Phase 3 randomised, open-label trial (NCT01774721) that directly compared dacomitinib against gefitinib, as first-line treatment for patients with EGFR-mutation +ve non-small-cell lung cancer (NSCLC), reported that dacomitinib significantly increased progression-free survival and overall survival compared to gefitinib [3]. Dacomitinib's use may be limited in NSCLC patients with brain metastases, as these patients were excluded from the trial because dacomitinib's ability to cross the blood-brain-barrier has not been fully confirmed. This is unlike osimertinib (another third-generation irreversible EGFR inhibitor) which has confirmed brain penetrance, and which does not appear to cause the same dose-limiting toxicities that afflict dacomitinib. In April 2018 both the FDA and EMA accepted regulatory submissions (New Drug Application and Marketing Authorization Application respectively) for review of dacomitinib as a therapy for metastatic NSCLC with EGFR-activating mutations. The FDA granted Priority Review status for the submitted NDA. SARS-CoV-2: A high-throughput screen identified potential anti-SARS-CoV-2 activity in cell infection assays [2]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Barf T, Kaptein A. (2012)
Irreversible protein kinase inhibitors: balancing the benefits and risks. J Med Chem, 55 (14): 6243-62. [PMID:22621397] |
|
2. Dittmar M, Lee JS, Whig K, Segrist E, Li M, Kamalia B, Castellana L, Ayyanathan K, Cardenas-Diaz FL, Morrisey EE et al.. (2021)
Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-Cov-2. Cell Rep, 35 (1): 108959. [PMID:33811811] |
|
3. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J et al.. (2017)
Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol, 18 (11): 1454-1466. [PMID:28958502] |