GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: NUC-1031
Compound class:
Synthetic organic
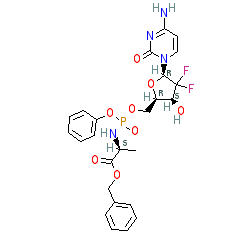
Comment: Acelerin is a candidate pro-drug. The proposed route to the active monophosphate form is depicted in Scheme 4 in [2]. Acerlarin is an analogue of gemcitabine, the main therapeutic used to treat pancreatic cancer. Acelarin is termed a ProTide, and structurally is gemcitabine with an attached phosphoramidate group. The aim of this chemical modification is to achieve improved cellular delivery of the activated drug, overcoming resistance mechanisms and protecting against enzymatic breakdown to toxic byproducts [2], and hopefully achieving better disease response. The chemical structure depicted here was drawn from [2] where acelerin is compound 6f.
Acelarin is the first ProTide to reach clinical trial and is only one member of a large development pipeline covering many nucleoside anti-metabolite anti-neoplastic drugs. |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Acelarin is in clinical trial in the ProGem1 phase 1 study, for the treatment of advanced solid tumours. |
Mechanism Of Action and Pharmacodynamic Effects  |
| To become an active antimetabolite, gemcitabine must be sequentially phosphorylated to the mono-, bi- and finally tri-phosphate forms (dFdCMP, dFdCDP and dFdCTP respectively). Adding the phosphoramidate effectively pre-phosphorylates the gemcitabine avoiding the first essential and rate-limiting phosphorylation step normally requiring deoxycytidine kinase (DCK, P277072]. In sum, the acelerin nucleoside has more effective antimetabolite activity than gemcitabine [1-2] and is already proving more efficacious in the clinical setting. |