GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
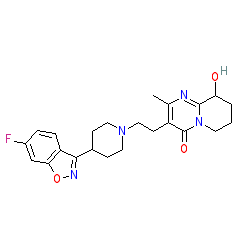
Synonyms: 9-hydroxyrisperidone | Invega® | Ro-76477 | Ro-92670
paliperidone is an approved drug (FDA (2006), EMA (2007))
Compound class:
Synthetic organic
Comment: Paliperidone is an atypical antipsychotic. Mechanistically it is a dopamine receptor and 5-HT2A receptor antagonist.

View more information in the IUPHAR Pharmacology Education Project: paliperidone |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Used to treat psychotic or manic symptoms of schizophrenia. In November 2014, the US FDA granted a supplemental New Drug Application (sNDA) for paliperidone palmitate (PubChem CID 9852746) as a treatment for schizoaffective disorder as either monotherapy or adjunctive therapy. This sNDA will be scrutinised under priority review, a mechanism whereby drugs may be approved more quickly, if they are found to offer significant improvement in the treatment of serious conditions, over currently available therapies. Following publication of results from a Phase 3 clinical trial [1], the FDA formally approved the use of paliperidone palmitate (trade named Invega Trinza) as a long-acting schizophrenia medication in May 2015. Invega Trinza has a dosing interval of three months. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |