GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
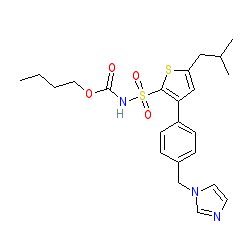
Synonyms: 3-[4-(1H-imidazol-1-ylmethyl)phenyl]-5-(2-methylpropyl)thiophene-2-[(N-butyloxylcarbamate)-sulphonamide] sodium salt | C21
Compound class:
Synthetic organic
Comment: Compound 21 is a first-in-class orally administered angiotensin AT2 receptor agonist. It prevents TNFα-induced and HFD-induced vascular inflammation in vitro and in vivo, indicating that its anti-atherosclerotic actions are due to vascular anti-inflammatory effects, mediated by AT2 receptors [2]. C21 is one of the chemical structures that are claimed in Vicore Pharma's patent US20180078529A1 [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04880642 | A Trial to Investigate Recovery From COVID-19 With C21 in Adult Subjects | Phase 3 Interventional | Vicore Pharma AB | ||
| NCT04452435 | Safety and Efficacy of C21 in Subjects With COVID-19 | Phase 2 Interventional | Vicore Pharma AB | Further evaluation in a Phase 3 study was undertaken after anaylsis from this Phase 2 trial found that there was a reduction in the requirement for oxygen at day 14 in hospitalised COVID-19 patients after 7 days of C21 treatment. However no C21-mediated reduction in CRP was detected compared to placebo. | 3 |
| NCT04533022 | Safety, Efficacy and Pharmacokinetics of C21 in Subjects With IPF | Phase 2 Interventional | Vicore Pharma AB | ||