GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Abbreviated name: CT
Synonyms: LS-173874 | Miacalcin Nasal® | thyrocalcitonin
calcitonin is an approved drug (FDA (1986))
Compound class:
Endogenous peptide in human, mouse or rat
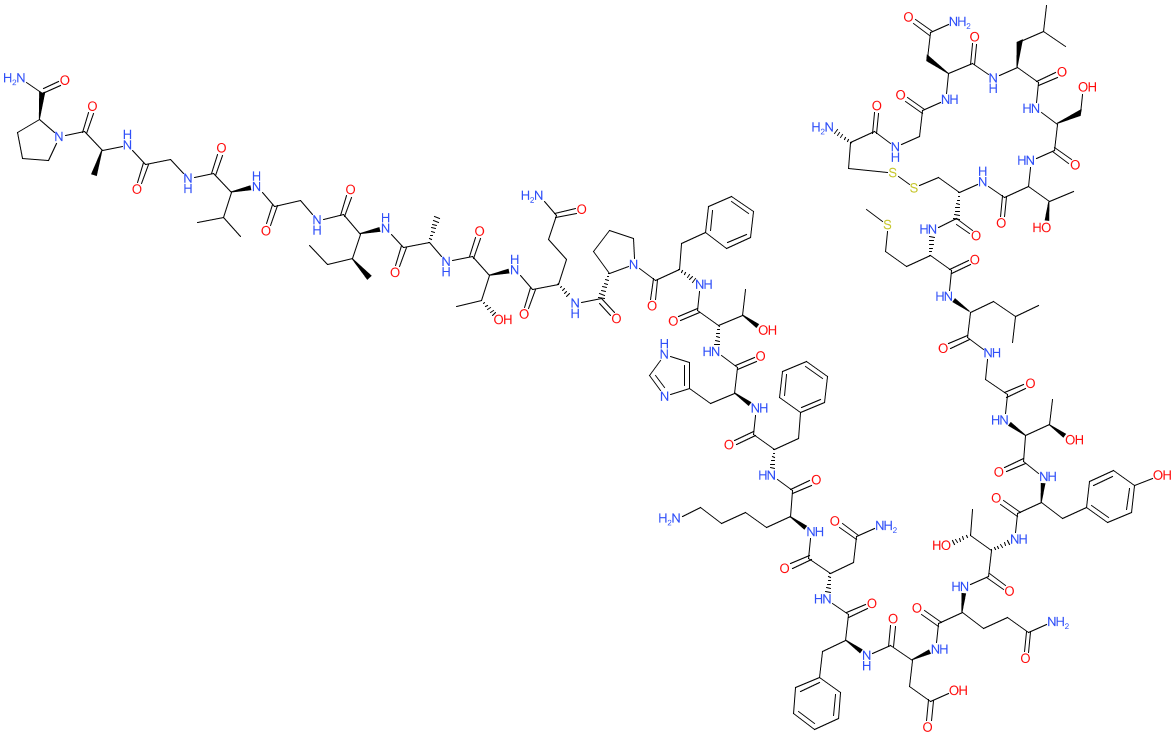
Comment: For an image and identifiers representing the chemical structure of human calcitonin, please see the PubChem entry linked to from this ligand page. The gene encoding human calcitonin also encodes two other isoforms: katacalcin and α-CGRP.
Species: Human
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: calcitonin |
|
|||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Calcitonin is used clinically to treat osteoporosis in people who have been in menopause for ≥5 years. |