GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
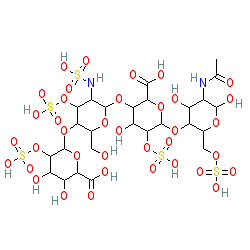
Synonyms: Clexane® | enoxaparin sodium | Lovenox® | PK-10169 | RP-54563
enoxaparin is an approved drug (FDA (1993), FDA (2016))
Compound class:
Synthetic organic
Comment: A Low-Molecular-Weight Heparin antithrombin-III activator. It consists of fractionated material from pig mucosa but the PubChem structure represents the major form.
Biosimilar drugs: The EMA approved enoxaparin biosimilars named Inhixa and Thorinane in 2016. 
View more information in the IUPHAR Pharmacology Education Project: enoxaparin |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Used to prevent and treat deep vein thrombosis or pulmonary embolism, and is given as a subcutaneous injection. SARS-CoV-2 and COVID-19: Based on scientific evidence that heparin is a potential target (attachment factor) for SARS-CoV [3] and other coronaviruses [1], and in reponse to the observed thrombotic pathology in COVID-19 patients, a clinical trial has been initiated in Italy (March 2020) to determine the efficacy of early treatment with enoxaparin in COVID-19 patients. |
Mechanism Of Action and Pharmacodynamic Effects  |
| A low molecular weight heparin; binds to and accelerates the activity of antithrombin III and ultimately prevents fibrin clot formation. |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |