GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
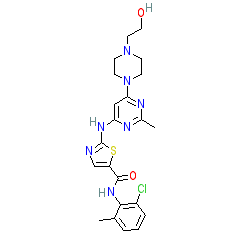
Synonyms: BMS 345825 | BMS 354825 | BMS 35482513 | Sprycel®
dasatinib is an approved drug (FDA & EMA (2006))
Compound class:
Synthetic organic
Comment: Dasatinib is a Type-1 kinase inhibitor and was first approved by the FDA in 2006.
A combination of dasatinib and quercetin has been investigated in animal models for senolytic activity [9,14,17]. Senolytic agents are intended to reduce the abundance of senescent cells as a mechanism to treat age-related diseases, reduce frailty and extend healthy lifespan [2,6-7,16]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: dasatinib |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Tyrosine kinase inhibitor approved for use in patients with chronic myelogenous leukemia (CML), including pediatric patients in the US as of November 2017 [5]. A Phase 2 clinical trial (NCT01467986) assessing dasatinib as a component of a multimodal molecular therapy to treat relapsed or refractory high-risk neuroblastoma is currently ongoing (Oct 2014). Snnolytic activity: Results of a phase 2 RCT to evaluate the effect of dasatinib plus quercetin on bone metabolism in postmenopausal women were published in 2024 [4]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04313634 | Targeting Cellular Senescence With Senolytics to Improve Skeletal Health in Older Humans | Phase 2 Interventional | Mayo Clinic | In this study dasatinib + quercetin was most effective (evaluating skeletal response in terms of change in the bone resorption marker C-terminal telopeptide of type 1 collagen) in participants with the highest senescent cell burden. | 4 |
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |