GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
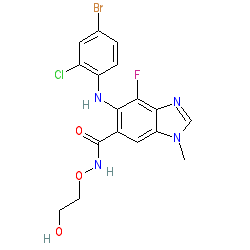
Synonyms: ARRY-142886 | ARRY-886 | AZD6244 | Koselugo® | NSC 741O78
selumetinib is an approved drug (FDA (2020))
Compound class:
Synthetic organic
Comment: Selumetinib is a selective, orally bioavailable MEK1/2 inhibitor [5].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Selumetinib (administered clinically as the hydrogen sulfate) was investigated for the treatment of various types of cancer, such as non-small cell lung cancer (NSCLC) and AIDS-related Kaposi's sarcoma. Click here to view ClinicalTrials.gov's list of registered selumetinib trials. In April 2015, the US FDA granted selumetinib orphan drug status as a treatment for uveal melanoma. Since July 2018 selumetinib has held EMA orphan designation for the treatment of neurofibromatosis type 1 (NF1). The first FDA approval of selumetinib was granted in April 2020, as a therapy for pediatric patients (≥1 year old), who have symptomatic NF1-associated, inoperable plexiform neurofibromas (PN) [3]. Koselugo was FDA approved for use in adults in November 2025 for this indication. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Selumetinib inhibits the MAPK kinases, MEK1 and MEK2 [6]. These kinases act in the MAPK/ERK pathway, downstream of BRAF and KRAS, two additional kinases which have been shown to carry activating mutations in some cancers [1]. Inhibition of MEK1/2 reduces abberant signalling in the growth factor-responsive MAPK/ERK signalling pathway in such cancer cells, and thereby reduces tumour growth. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01362803 | AZD6244 Hydrogen Sulfate for Children With Nervous System Tumors | Phase 1/Phase 2 Interventional | National Institutes of Health Clinical Center (CC) | Selumetinib was approved as the first oral monotherapy for pediatric patients with NF1-related plexiform neurofibromas. Regulatory approval was based on results from the SPRINT (NCT01362803) clinical trial which showed that selumetinib induced durable tumour shrinkage and clinical benefit in these patients. | 4 |