GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: DRG-0115 | Mycapssa® | Sandostatin® | SMS 201,995
octreotide is an approved drug (FDA (1988), EMA (2022))
Compound class:
Peptide
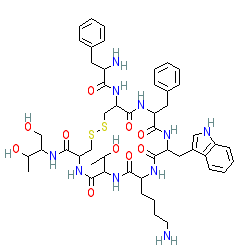
Comment: Octreotide is a somatostatin mimetic.
As with many peptide molecules there is ambiguity around representations of the stereochemistry of octreotide. The chemical structure shown here does not specifiy stereochemistry and so may differ from representations on other chemical information resources in the Database Links table. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Octreotide is used for the treatment of growth hormone producing tumours and pituitary tumours secreting thyroid stimulating hormone, and is used in nuclear medicine imaging of somatostatin receptor-expressing neuroendocrine tumours. In June 2020, an octreotide biosimilar (Mycapssa®, Amryt Pharmaceuticals) was FDA approved as long-term maintenance treatment for acromegaly patients who have responded to and tolerated treatment with octreotide or lanreotide. An equivalent approval for Mycapssa® was issued by the EMA in 2022. |