GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
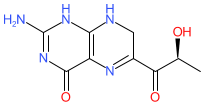
Synonyms: CNSA-001 [1] | L-sepiapterin | PTC-923 | PTC923 | Sephience®
sepiapterin is an approved drug
Compound class:
Synthetic organic
Comment: Sepiapterin (PTC923) is an orally bioavailable phenylalanine hydroxylase (PAH) activator. It is a precursor of the PAH cofactor BH4 (tetrahydrobiopterin/sapropterin).
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Abell TL, Garcia LM, Wiener GJ, Wo JM, Bulat RS, Smith N. (2021)
Effect of Oral CNSA-001 (sepiapterin, PTC923) on gastric accommodation in women with diabetic gastroparesis: A randomized, placebo-controlled, Phase 2 trial. J Diabetes Complications, 35 (9): 107961. [PMID:34176722] |
|
2. Bratkovic D, Margvelashvili L, Tchan MC, Nisbet J, Smith N. (2022)
PTC923 (sepiapterin) lowers elevated blood phenylalanine in subjects with phenylketonuria: a phase 2 randomized, multi-center, three-period crossover, open-label, active controlled, all-comers study. Metabolism, 128: 155116. [PMID:34973284] |
|
3. Lamb YN. (2025)
Sepiapterin: First Approval. Drugs, [Epub ahead of print]. [PMID:41091365] |
|
4. Muntau AC, Longo N, Ezgu F, Schwartz IVD, Lah M, Bratkovic D, Margvelashvili L, Kiykim E, Zori R, Campistol Plana J et al.. (2024)
Effects of oral sepiapterin on blood Phe concentration in a broad range of patients with phenylketonuria (APHENITY): results of an international, phase 3, randomised, double-blind, placebo-controlled trial. Lancet, 404 (10460): 1333-1345. [PMID:39368841] |
|
5. Smith N, Longo N, Levert K, Hyland K, Blau N. (2019)
Phase I clinical evaluation of CNSA-001 (sepiapterin), a novel pharmacological treatment for phenylketonuria and tetrahydrobiopterin deficiencies, in healthy volunteers. Mol Genet Metab, 126 (4): 406-412. [PMID:30922814] |