GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: CNSA-001 [1] | L-sepiapterin | PTC-923 | PTC923 | Sephience®
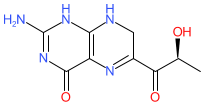
sepiapterin is an approved drug
Compound class:
Synthetic organic
Comment: Sepiapterin (PTC923) is an orally bioavailable phenylalanine hydroxylase (PAH) activator. It is a precursor of the PAH cofactor BH4 (tetrahydrobiopterin/sapropterin).
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Sepiapterin (PTC923) was advanced to clinical trials to evaluate safety and efficacy as a treatment to reduce neurotoxic phenylalanine accumulation (hyperphenylalaninemia) in patients with phenylketonuria [2,5]. The EU EMA approved the drug in April 2025 for this indication [3], with US FDA approval covering all subtypes of the disease following in July 2025. Sepiapterin is used alongside dietary phenylalanine restriction. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05099640 | A Study of PTC923 in Participants With Phenylketonuria | Phase 3 Interventional | PTC Therapeutics | The phase 3 APHENITY trial was pivotal in sepiapterin's clinical approval. | 4 |
| NCT06302348 | A Study of Sepiapterin in Participants With Phenylketonuria (PKU) | Phase 3 Interventional | PTC Therapeutics | ||