GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound 22 [PMID: 36655128] | CRN-00808 | CRN00808 | Palsonify®
paltusotine is an approved drug
Compound class:
Synthetic organic
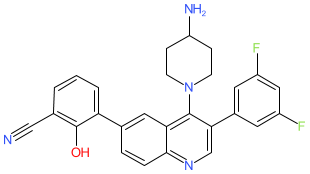
Comment: Paltusotine (CRN00808) is a small molecule, orally bioavailable somatostatin receptor type 2 (SST2) agonist [5-6]. It acts to inhibit production of growth hormone and insulin-like growth factor 1.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Paltusotine (CRN00808) was advanced to clinical evaluation to determine safety and efficacy for the treatment of acromegaly [1,3-4], and neuroendocrine tumour-associated carcinoid syndrome [2]. First approval was granted by the FDA in September 2025, to treat acromegaly. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT07087054 | Carcinoid Syndrome Efficacy Study Featuring an Oral Daily Paltusotine Regimen | Phase 3 Interventional | Crinetics Pharmaceuticals Inc. | ||
| NCT05192382 | A Study to Evaluate the Safety and Efficacy of Paltusotine for the Treatment of Acromegaly (PATHFNDR-2) | Phase 3 Interventional | Crinetics Pharmaceuticals Inc. | ||
| NCT04837040 | A Study to Evaluate the Safety and Efficacy of Paltusotine for the Treatment of Acromegaly | Phase 3 Interventional | Crinetics Pharmaceuticals Inc. | ||