GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
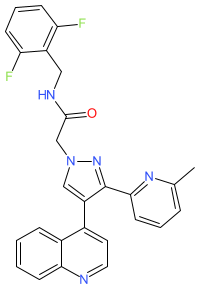
Synonyms: AGMB-129 | Example 26 [WO2021105317A1] | ORG-129
Compound class:
Synthetic organic
Comment: The chemical structure for ontunisertib was obtained from WHO INN proposed list 132. This matched a structure claimed in patent WO2021105317A1 (Origo Biopharma) [1]. This patent claims the compound as a transforming growth factor β receptor 1 (TGFβR1)/ALK5 inhibitor. This is likely one of Origo's lead assets ORG-129 (a gastrointestinal tract restricted inhibitor for fibrostenotic Crohn's disease) or ORG-447 (a lung-restricted inhibitor for idiopathic pulmonary fibrosis), most probably ORG-129 as this has progressed to clinical trial.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Selectivity at catalytic receptors | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||