GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
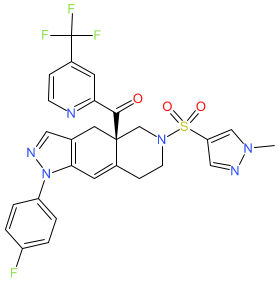
Synonyms: compound 7 [PMID: 28368581] | CORT-125134 | CORT125134
Compound class:
Synthetic organic
Comment: Relacorilant (CORT125134; Corcept Therapeutics) is an orally bioavailable selective glucocorticoid receptor (GR) modulator (antagonist) [4]. It was originally developed as a treatment for Cushing syndrome, and was subsequently identified as offering antineoplastic potential.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Relacorilant (CORT125134) is being investigated for efficacy as a treatment for Cushing syndrome (endogenous hypercortisolism) [6], and for antitumour activity [1,5]. In 2018 the FDA granted orphan drug designation for use as a Cushing syndrome therapy and to treat pancreatic cancer . |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02804750 | Study to Evaluate CORT125134 in Participants With Cushing's Syndrome | Phase 2 Interventional | Corcept Therapeutics | ||
| NCT03776812 | Study of Relacorilant in Combination With Nab-Paclitaxel for Patients With Recurrent Platinum-Resistant Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | Phase 2 Interventional | Corcept Therapeutics | ||
| NCT04329949 | Study of Relacorilant in Combination With Nab-Paclitaxel in Patients With Metastatic Pancreatic Ductal Adenocarcinoma | Phase 3 Interventional | Corcept Therapeutics | 1 | |
| NCT05257408 | Relacorilant in Combination With Nab-Paclitaxel in Advanced, Platinum-Resistant, High-Grade Epithelial Ovarian, Primary Peritoneal, or Fallopian-Tube Cancer | Phase 3 Interventional | Corcept Therapeutics | 2,5 | |
| NCT03697109 | A Study of the Efficacy and Safety of Relacorilant in Patients With Endogenous Cushing Syndrome | Phase 3 Interventional | Corcept Therapeutics | The GRACE study | |