GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
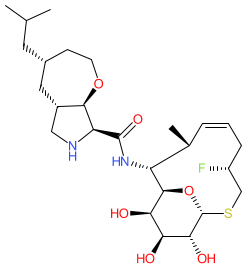
Comment: BT-33 is a fluorinated macrobicyclic oxepanoprolinamide antibacterial compound with broad-spectrum activity against multidrug-resistant (MDR) bacteria [2]. It was discovered through a collaboration between the University of Illinois at Chicago and Harvard University and is one of the chemical structures claimed in patent WO2023205206A1 [1].
|
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| BT-33 is reported to have improved potency against Gram-positive and Gram-negative bacteria relative to earlier oxepanoprolinamide antibacterials iboxamycin and cresomycin [2]. |