GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: GV118819
Compound class:
Synthetic organic
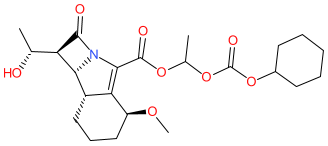
Comment: Sanfetrinem cilexetil is the orally bioavailable prodrug of sanfetrinem [1]. It is under clinical development as a treatment for tuberculosis (TB).
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Sanfetrinem cilexetil was developed by GlaxoSmithKline in the 1990s and progressed to clinical evaluation as a treatment for bacterial infections of the upper respiratory tract. Its development was halted based on commercial considerations. More recently, drug repurposing efforts have identified the potential of sanfetrinem cilexetil as a treatment for TB [2]. The drug has completed a Phase 2 trial to evaluate safety, tolerability, pharmacokinetics, and early bactericidal activity (EBA) in adult participants with rifampicin-sensitive pulmonary TB (NCT05388448). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05388448 | EBA, Safety and Tolerability of Sanfetrinem Cilexetil | Phase 2 Interventional | TASK Applied Science | ||