GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: MMV1634402 | PMX30063
Compound class:
Synthetic organic
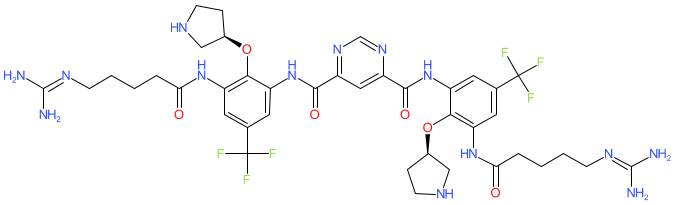
Comment: Brilacidin is a small molecule mimetic of defensin, an antimicrobial peptide (also referred to as a host defence peptide) [6]. Brilacidin has anti-inflammatory properties and exhibits broad-spectrum antimicrobial activity, including antibacterial [4,6], antimalarial [1], antifungal [5], and antiviral [2-3]. Brilacidin (MMV1634402) was one of the chemotypes included in the Medicines for Malaria Pandemic Response Box.
The Malaria tab on this ligand page provides additional curator comments of relevance to the Guide to MALARIA PHARMACOLOGY. |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Brilacidin is being developed by Innovation Pharma to target multiple indications. Phase 2 trials for acute bacterial skin and skin structure infections (ABSSSI) (NCT02052388), oral mucositis in patients undergoing chemoradiation for treatment of head and neck cancer (NCT02324335), and patients hospitalised due to COVID-19 (NCT04784897) have all been completed. The US FDA has granted Qualified Infectious Disease Product (QIDP) designation to brilacidin as a treatment for ABSSSI and Fast Track designation for the prevention of oral mucositis and for the treatment of COVID-19. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02052388 | Efficacy and Safety Study of Brilacidin to Treat Serious Skin Infections | Phase 2 Interventional | Innovation Pharmaceuticals, Inc. | ||
| NCT02324335 | Study of the Effects of Brilacidin Oral Rinse on Radiation-induced Oral Mucositis in Patients With Head and Neck Cancer | Phase 2 Interventional | Innovation Pharmaceuticals, Inc. | ||
| NCT04784897 | A Study to Evaluate the Efficacy and Safety of Brilacidin in Hospitalized Participants With COVID-19 | Phase 2 Interventional | Innovation Pharmaceuticals, Inc. | ||