GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound (S)-058 [US20230322711] | KO-2806 | KO2806
Compound class:
Synthetic organic
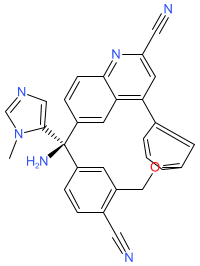
Comment: We have matched the chemical structure for the INN darlifarnib to Kura Oncology's farnesyl transferase inhibitor clinical candidate KO-2806 [1]. The structure is one of those claimed in patent US20230322711A1 [2]. KO-2806 is proposed as an agent for use in combination with RAS inhibitors, to overcome resistance to these drugs. KO-2806 functions to inhibit farnesylation and subsequent activation of mTORC1 [1]. It will also block farnesylation of the CAAX motif on RAS oncogenes that is essential for RAS anchoring to the cell membrane where (proliferative) RAS extracellular signals are transduced [2].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
Download 2D Structure  |
|
| Canonical SMILES | Download |
| Isomeric SMILES | Download |
| InChI standard identifier | Download |
| InChI standard key | Download |
Molecular structure representations generated using Open Babel