GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
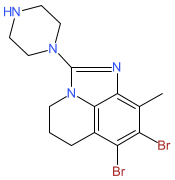
Synonyms: RVU120 | SEL120 | SEL120-34A
Compound class:
Synthetic organic
Comment: Romaciclib (RVU120; SEL120-34A) is an ATP-competitive and selective cyclin-dependent kinase 8 (CDK8) inhibitor [1]. The functions of CDK8 and its paralog CDK19 are implicated in sustained proliferation and viability of cancer cells through modulation of oncogenic transcriptional processes. Inhibition of CDK8 function is a focus of current anti-cancer therapeutic development, particularly in relation to acute myeloid leukemia.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Ryvu Therapeutics are evaluating the safety and efficacy of romaciclib (as RVU120) as a treatment for myelodysplastic syndromes and acute myeloid leukemia (AML). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT06397313 | RVU120 in Patients with Intermediate or High-risk, Primary or Secondary Myelofibrosis | Phase 2 Interventional | Ryvu Therapeutics SA | ||
| NCT06268574 | Safety and Efficacy of RVU120 for Treatment of Relapsed/Refractory AML | Phase 2 Interventional | Ryvu Therapeutics SA | ||
| NCT06243458 | RVU120 for Treatment of Anemia in Patients With Lower-risk Myelodysplastic Neoplasms | Phase 2 Interventional | GCP-Service International West GmbH | ||
| NCT06191263 | Safety and Efficacy of RVU120 Combined with Venetoclax for Treatment of Relapsed/Refractory AML | Phase 2 Interventional | Ryvu Therapeutics SA | ||