GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
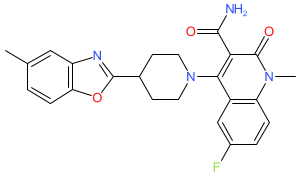
Synonyms: Example 298 [US11998539B2]
Compound class:
Synthetic organic
Comment: Pasodacigib is the INN for a diacylglycerol kinase (DGK) inhibitor. A structural match via PubChem reveals that it is one of the claims in Bayer patent US11998539B2. There are two diacylglycerol kinase inhibitors (one each for DGKα and DGKζ) listed in the oncology section of Bayer's development pipeline page [1], but no internal code numbers have been ascribed to these. However patent US11998539B2 claims DGKα inhibitors, so it would be reasonable to assume pasodacigib to be a DGKα inhibitor. Further, the record for Bayer-led clinical trial (NCT05858164) lists the drug intervention as BAY2862789, which is recorded by the US National Cancer Institute's drug definition service as a DGKα inhibitor [2]. We must wait for formal disclosure of BAY2862789's structure before equating the INN to BAY2862789.
DGK inhibition mediates T cell activation, and this mechanism is being leveraged to promote a clinically beneficial anti-tumour immune response. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Selectivity at enzymes | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||