GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound T4-B [US20220106319A1] | GGW-101 | GGW101
Compound class:
Synthetic organic
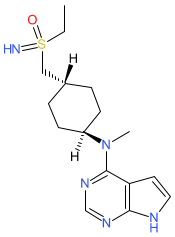
Comment: Lirucitinib is the INN for a Janus tyrosine kinase inhibitor with anti-inflammatory potential. The INN record specifies the (R)-enantiomer of the molecule. The chemical structure is claimed in Felicamed Biotechnology's patent US20220106319A1 [1]. An internet search reveals that lirucitinib was approved in China (by the Ministry of Agriculture and Rural Affairs) in 2024 for veterinary use, to treat pruritic skin diseases in pet dogs. There is no indication that lirucitinib will be tested for use in humans.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
Download 2D Structure  |
|
| Canonical SMILES | Download |
| Isomeric SMILES | Download |
| InChI standard identifier | Download |
| InChI standard key | Download |
Molecular structure representations generated using Open Babel