|
Synonyms: acetylisovaleryltylosin | Aivlosin® (veterinary)
Comment: Tylvalosin is a 16-membered macrolide antibacterial compound derived by biotransformation of tylosin by Streptomyces thermotolerans [ 1-2]. It was developed for use in veterinary medicine.
|
|
2D Structure
|
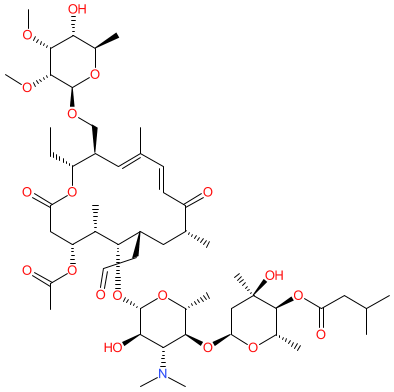
|
Physico-chemical Properties
|
|
|
Hydrogen bond acceptors
|
20
|
|
Hydrogen bond donors
|
3
|
|
Rotatable bonds
|
19
|
|
Topological polar surface area
|
250.81
|
|
Molecular weight
|
1042.26
|
|
XLogP
|
3.17
|
|
No. Lipinski's rules broken
|
3
|
Generated using the Chemistry Development Kit (CDK) (Willighagen EL et al. Journal of Cheminformatics vol. 9:33. 2017, doi:10.1186/s13321-017-0220-4; https://cdk.github.io/)
|
SMILES / InChI / InChIKey
|
|
|
Canonical SMILES
|
CC[C@@H]1[C@H](/C=C(\C)/C=C/C(=O)[C@H](C)C[C@H](CC=O)[C@@H]([C@@H](C)[C@@H](CC(=O)O1)OC(=O)C)O[C@H]2[C@@H]([C@H]([C@@H]([C@@H](C)O2)O[C@H]3C[C@](C)([C@H]([C@H](C)O3)OC(=O)CC(C)C)O)N(C)C)O)CO[C@H]4[C@@H]([C@@H]([C@@H]([C@@H](C)O4)O)OC)OC
|
|
Isomeric SMILES
|
CC[C@@H]1[C@H](/C=C(/C=C/C(=O)[C@@H](C[C@@H]([C@@H]([C@H]([C@@H](CC(=O)O1)OC(=O)C)C)O[C@H]2[C@@H]([C@H]([C@@H]([C@H](O2)C)O[C@H]3C[C@@]([C@H]([C@@H](O3)C)OC(=O)CC(C)C)(C)O)N(C)C)O)CC=O)C)\C)CO[C@H]4[C@@H]([C@@H]([C@@H]([C@H](O4)C)O)OC)OC
|
|
InChI
|
InChI=1S/C53H87NO19/c1-16-38-36(26-65-52-49(64-15)48(63-14)44(60)31(7)67-52)22-28(4)17-18-37(57)29(5)23-35(19-20-55)46(30(6)39(69-34(10)56)24-41(59)70-38)73-51-45(61)43(54(12)13)47(32(8)68-51)72-42-25-53(11,62)50(33(9)66-42)71-40(58)21-27(2)3/h17-18,20,22,27,29-33,35-36,38-39,42-52,60-62H,16,19,21,23-26H2,1-15H3/b18-17+,28-22+/t29-,30+,31-,32-,33+,35+,36-,38-,39-,42+,43-,44-,45-,46-,47-,48-,49-,50+,51+,52-,53-/m1/s1
|
|
InChI Key
|
KCJJINQANFZSAM-HZDSEHBESA-N
|
Generated using the Chemistry Development Kit (CDK) (Willighagen EL et al. Journal of Cheminformatics vol. 9:33. 2017, doi:10.1186/s13321-017-0220-4; https://cdk.github.io/)
|
|







