GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
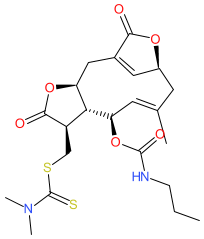
Comment: This small molecule is a prodrug that produces an active compound that is a covalent inhibitor of thioredoxin reductase 1 (TrxR1) [1].
|
|
|||||||||||||||||||||||||||||||||||