GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Draxxin® (veterinary) | tulathromycin A
Compound class:
Synthetic organic
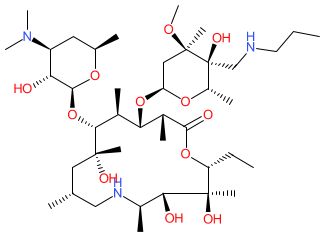
Comment: Tulathromycin is a macrolide (triamilide subclass) antibacterial that was developed for use in veterinary medicine [3]. It consists of an equilibrated mixture of a 15-membered and a 13-membered ring component in a 9:1 ratio [2]. We show the structure for tulathromycin A, the 15-membered isomer.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Andersen NM, Poehlsgaard J, Warrass R, Douthwaite S. (2012)
Inhibition of protein synthesis on the ribosome by tildipirosin compared with other veterinary macrolides. Antimicrob Agents Chemother, 56 (11): 6033-6. [PMID:22926570] |
|
2. Gáler D, Hessong S, Beato B, Risk J, Inskeep P, Weerasinghe C, Schneider RP, Langer C, LaPerle J, Renouf D et al.. (2004)
An analytical method for the analysis of tulathromycin, an equilibrating triamilide, in bovine and porcine plasma and lung. J Agric Food Chem, 52 (8): 2179-91. [PMID:15080618] |
|
3. Norcia LJ, Silvia AM, Santoro SL, Retsema J, Letavic MA, Bronk BS, Lundy KM, Yang B, Evans NA, Hayashi SF. (2004)
In vitro microbiological characterization of a novel azalide, two triamilides and an azalide ketal against bovine and porcine respiratory pathogens. J Antibiot (Tokyo), 57 (4): 280-8. [PMID:15217193] |
|
4. Villarino N, Brown SA, Martín-Jiménez T. (2013)
The role of the macrolide tulathromycin in veterinary medicine. Vet J, 198 (2): 352-7. [PMID:24268476] |