GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Draxxin® (veterinary) | tulathromycin A
Compound class:
Synthetic organic
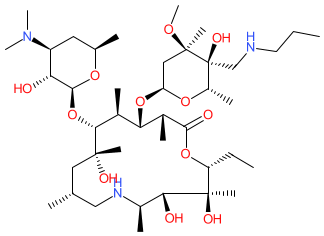
Comment: Tulathromycin is a macrolide (triamilide subclass) antibacterial that was developed for use in veterinary medicine [3]. It consists of an equilibrated mixture of a 15-membered and a 13-membered ring component in a 9:1 ratio [2]. We show the structure for tulathromycin A, the 15-membered isomer.
|
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Tulathromycin demonstrates improved activity compared to tilmicosin, against field isolates of Gram-negative bacteria that are the common cause of respiratory tract infections in animals [3]. The MIC90 for antibacterial activity against Mannheimia haemolytica and Pasteurella multocida is 1 μg/ml in vitro [3]. Tulathromycin inhibits bacterial protein synthesis with an IC50 of 0.26 ± 0.05 μM [1]. |