GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
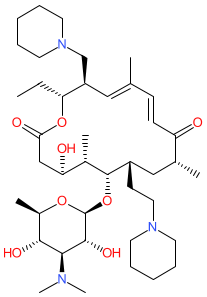
Synonyms: Zuprevo® (veterinary)
Compound class:
Synthetic organic
Comment: Tildipirosin is a 16-membered macrolide antibacterial compound derived from tylosin. It was developed for use in veterinary medicine.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Mechanism Of Action and Pharmacodynamic Effects  |
| A study using a combination of chemical footprinting and structure modeling has shown that tildipirosin binds in the polypeptide exit tunnel adjacent to the peptidyl transferase centre (PTC) of the bacterial 50S ribosomal subunit, suggesting that it inhibits bacterial protein synthesis by interfering with prolongation of the nascent polypeptide chain [2]. |